Stryker Corp Continues to Deal with Fallout of Defective Medical Equipment
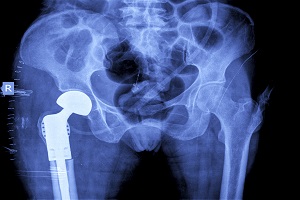
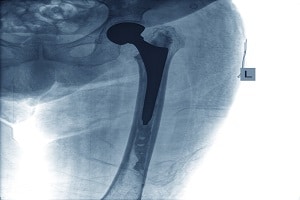
The fallout surrounding the recall of Stryker Corp’s Rejuvenate and ABG II Modular Hip Implants continues despite the announcement of a major settlement agreement in November. The November 3rd settlement offer was an attempt to resolve thousands of claims filed against the company by patients who experienced severe pain and complications after receiving a Stryker …